For hydrogen chloride, the molar mass is 1.007 + 35.453 = 36.460 g/mol. 36.46 grams is the mass of one mole of hydrogen chloride. For glucose, the molar mass is 72.0642 + 12.084 + 95.9964 = 180.1446 g/mol. 180.14 grams is the mass of one mole of glucose. Advertisement. Calculator, Practice Problems, and Answers.. It is also known as hydrogen chloride or muriatic acid. When hydrogen chloride is dissolved in water HCl is formed. It is a simple diatomic molecule. The hydrogen and chlorine atom are connected with a single covalent bond.. Molecular Weight/ Molar Mass: 36.458 g/mol: Appears: Transparent liquid: Boiling Point: Depends on the concentration.
H2o molar mass fasshark
[Solved] A compound with a molar mass of 78.0 g/mol is found to contain... Course Hero

Printable Periodic Table Molar Mass Periodic Table Timeline

3 Ways to Calculate Molar Mass wikiHow

Molar Mass Worksheet Easy Hard Science

How to Calculate Molar Mass 7 Steps (with Pictures) wikiHow
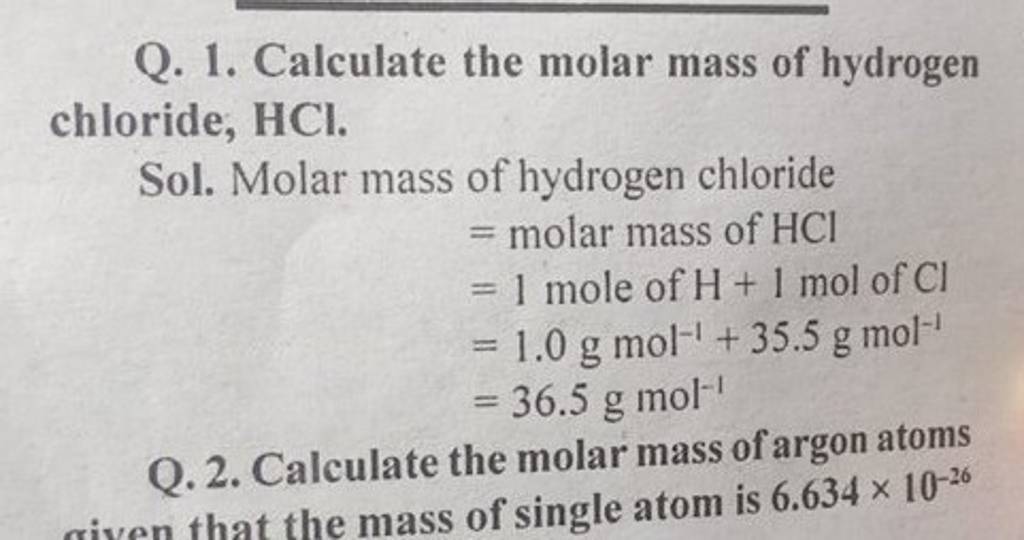
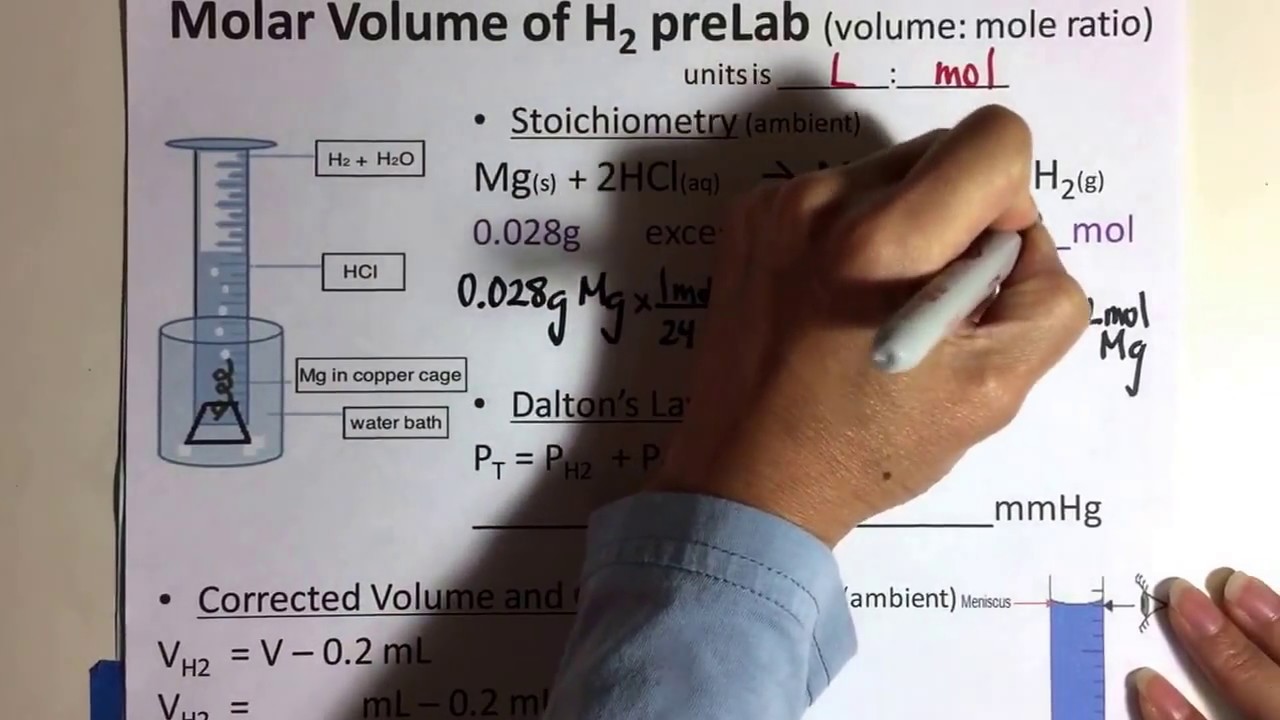
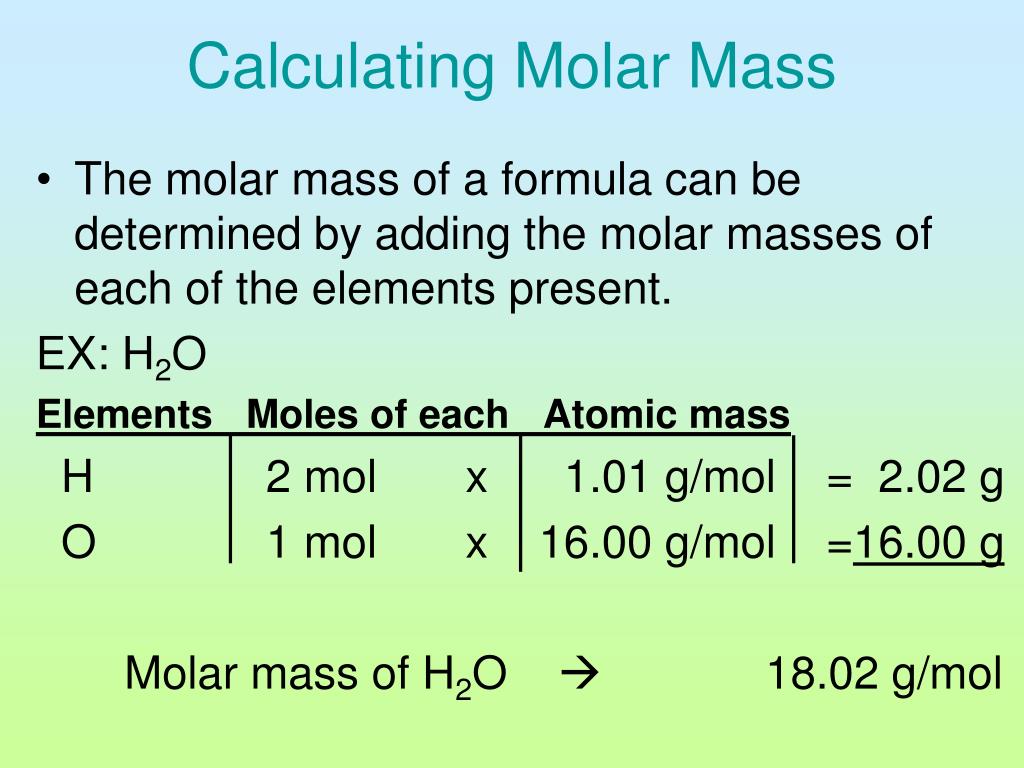
Q. 1. Calculate the molar mass of hydrogen chloride, HCl. Sol. Molar mass..
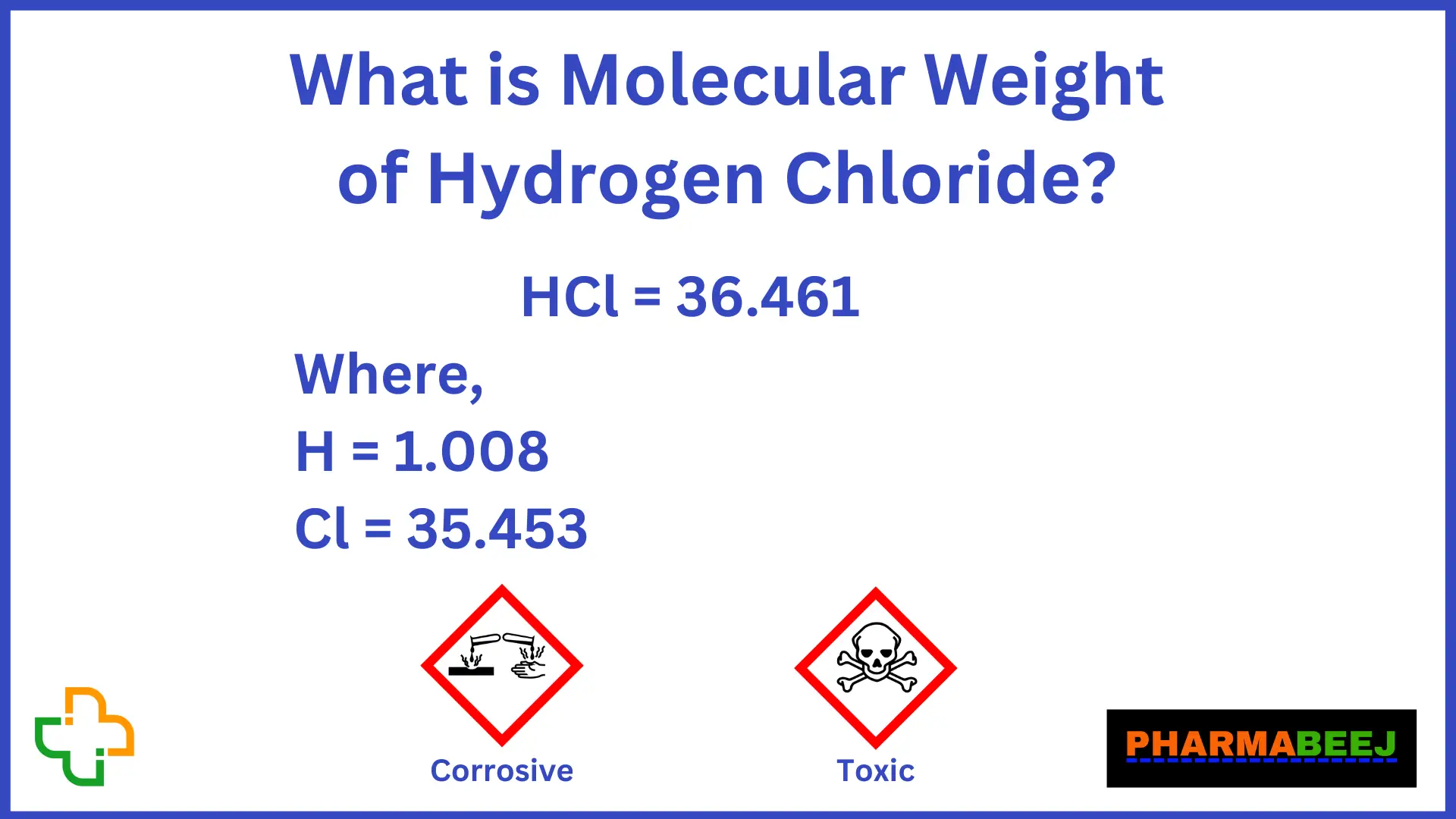
What Is Molecular Formula Of Hydrogen Chloride? Pharmabeej

Hydrogen Gas Molar Mass Of Hydrogen Gas
Chemistry Atomic Mass and Molecular Mass Atoms and Molecules Part 5 English YouTube
Al molar mass htpastor

What is Molar Mass

CHEMISTRY 101 Molar mass of a compound YouTube
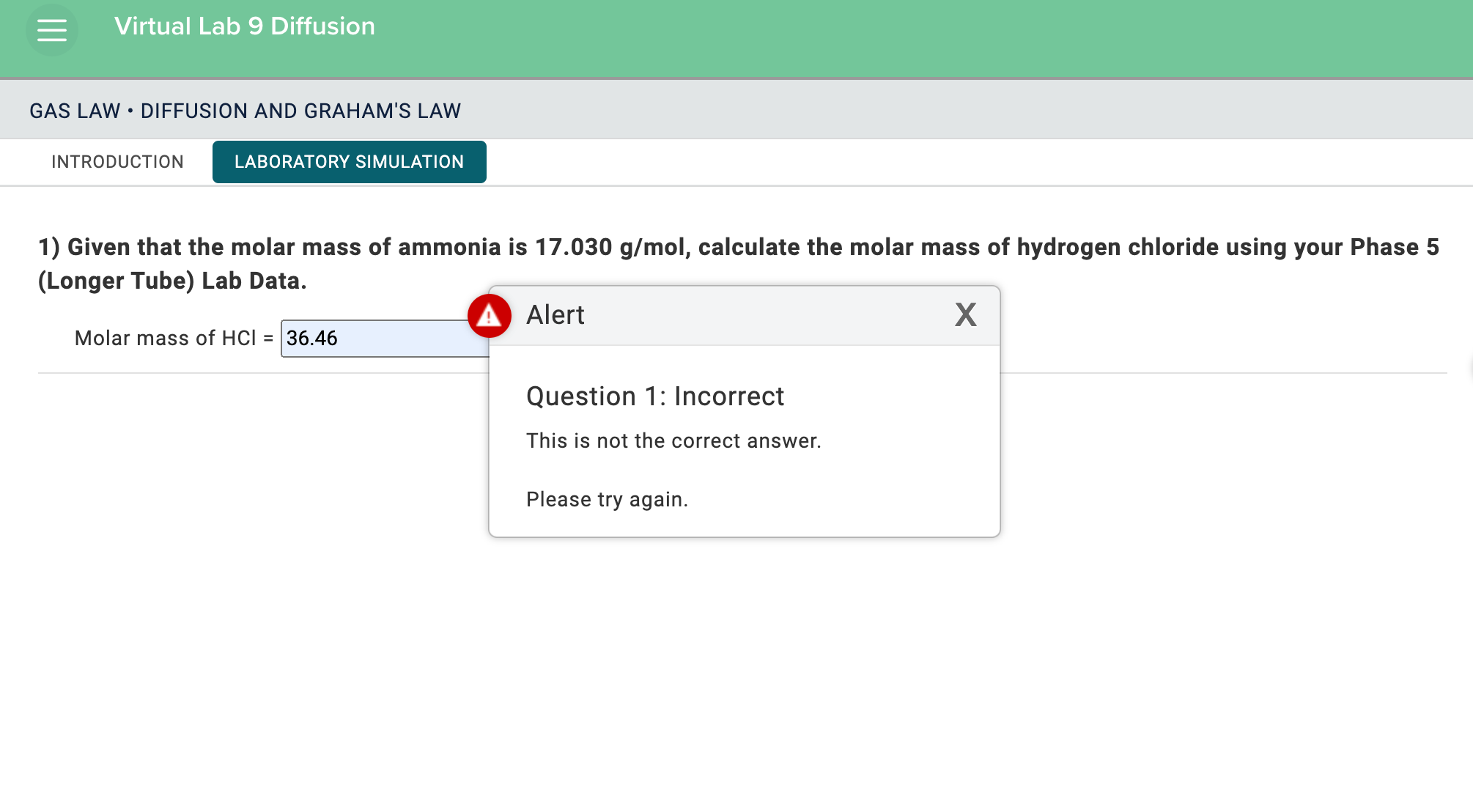
Solved Given that the molar mass of ammonia is 17.030 g/mol,
Nh4cl Molar Mass

SOLVED Calculate the mass in grams of hydrogen chloride, HCI produced when 26 L of molecular

PPT MOLAR MASS PowerPoint Presentation, free download ID2674198
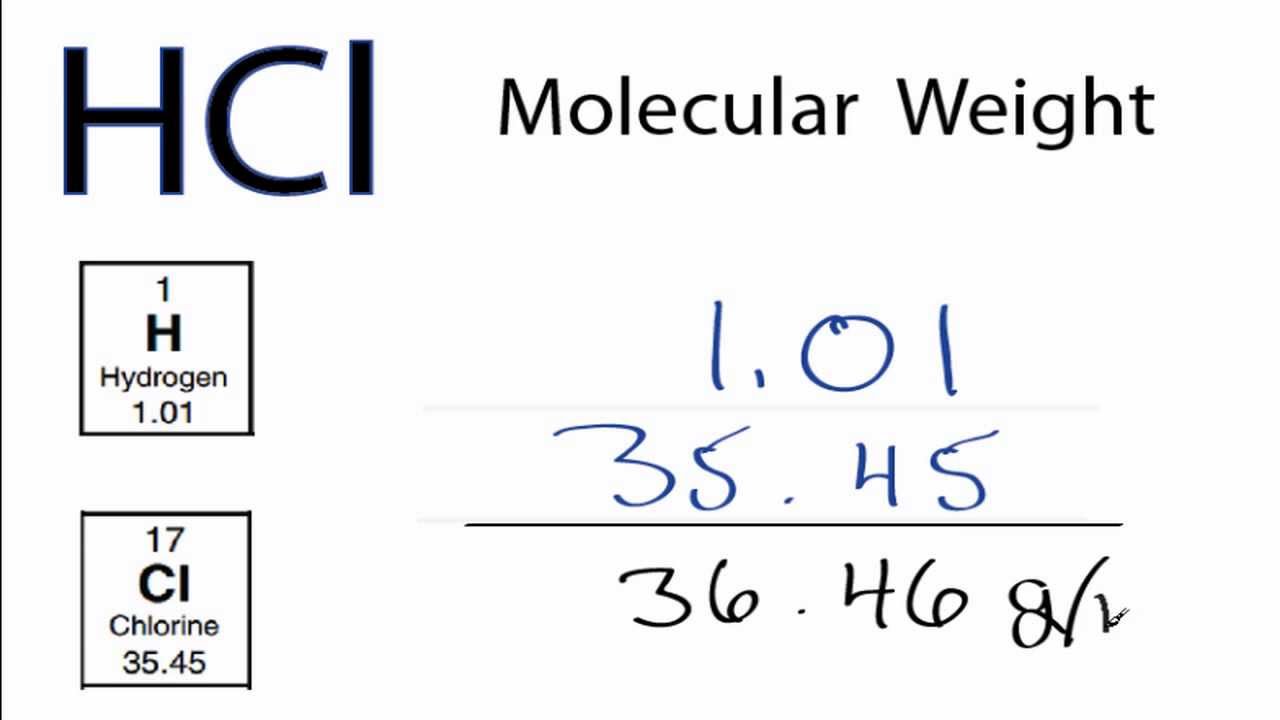
Molar Mass / Molecular Weight of HCl Hydrochloric acid YouTube

How to Find Molecular Mass

Molar Mass of NH4Cl (Ammonium chloride) YouTube
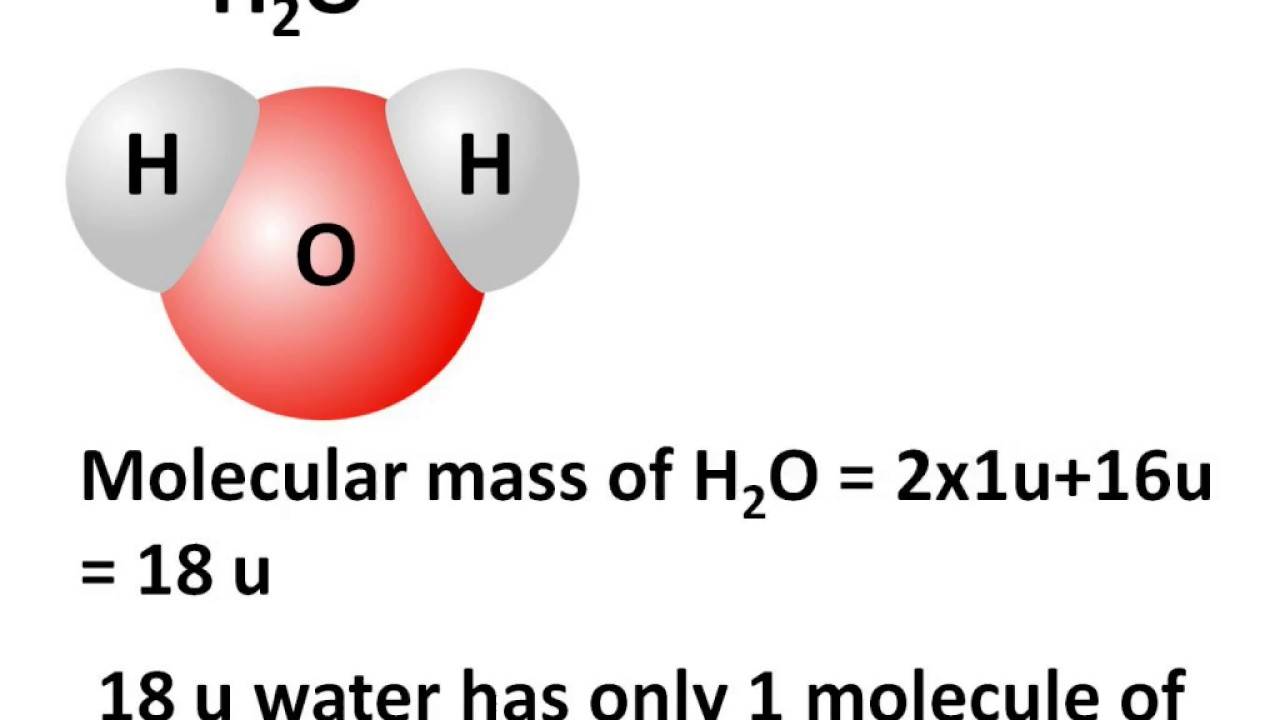
Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in HCl: Molar Mass (g/mol) H (Hydrogen) 1 × 1.00794 = 1.00794. Cl (Chlorine) 1 × 35.453 = 35.453. 4. Sum Each Element's Mass. Finally, add together the total mass of each element to get the molar mass of HCl:. The molar mass of a substance is defined as the mass of 1 mol of that substance,. (potassium chloride) NaCN (sodium cyanide) H 2 S (hydrogen sulfide) NaN 3 (sodium azide). One mole of H 2 has 6.022 × 10 23 hydrogen atoms. The molecular mass of H 2 O is 18.0 amu. The formula mass of benzene is 78 amu.